Pest Risk and Preventive Conservation Theory
The majority of this document was created by the Australian Museum’s Collection Care and Conservation team.
What is a pest? A pest is a living organism can cause damage to a material or structure. Any animal not acting as a pest but within the space may be an indicator of environmental conditions or pathways of ingress in the building. Any organism subsisting on any of these animals is considered a predator specimen. Other animals in your store that do not fit these descriptions is considered a nuisance. Museum Integrated Pest Management considers them all under the umbrella of ‘pest,’ and it is important when identifying pests that you categorise them to understand their risk.
An integrated pest management plan is a bespoke document created and used by institutions to strategise their preventive approach to pest management. The fundamental aim is to reduce pest risk and damage to collections, minimising the need for chemical solutions. Some organisations will only use pesticides when there is an outbreak. However, in certain environments, such as tropical regions, it can be almost impossible to rely solely on non-chemical treatments to control pest outbreaks. Therefore, IPM plans must be adapted to each institution’s location and specific climate. A thorough and well-maintained plan can reduce organisation costs and result in less damage to collection materials. An IPM plan should be reviewed regularly depending on the collection risk to pest infestation. For high-risk organisations, this might be yearly; conducting a thorough review for lower risk, this may be every 5 to 10 years.
Preventive conservation takes a risk management approach to collection care which in this page is summarised as follows:
- Define the risk: Pests are more likely to damage organic material in collections. However, their presence can also cause staining and damage to non-organic material. To understand the risk to the individual collection, surveying the site, buildings and rooms is important. Identify rooms with high-risk collections and areas where pest may flourish such as kitchens and food storage.
- Levels of control: The levels of control are methodologies that can be put in place to reduce the risk. These can be enacted at the site, building, room, or object levels
- Types of controls: form the principles of IPM. These controls and steps should be enacted at different levels to mitigate the pest risk.
Importantly, the preventive conservation approach to IPM reduces the risk of pests occurring in a collection. At its heart is understanding insect biology: all insects need four things to survive and thrive in collections; warmth, moisture, quiet, and food.
Mapping the Building
Crucial to creating an IPM plan is defining your risk of pest infestation. A useful tool to start this is creating a map of the building using existing floorplans or drawing a simple sketch. Facilities or Building Services teams may already have these available. It is important during this process to understand the function of the room and how it is currently being used.
Things to note while you are mapping:
- Use the IPM survey checklist spreadsheet in the resources section to note any of the building features that increase pest risk
- Map where collections are and if they are enclosed or opened
- Map garden areas in relation to the building and any external collections
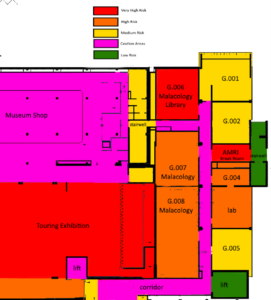
Figure 1: Section of a floor plan from the Australian Museum. The Museum uses a colour coded risk zone system for mapping higher risk areas. The risk for areas is determined by if a room is internal or external, the material in the room and purpose of the space.
Approaching the map will allow you to define and rate the pest risk by understanding the limits of your site, the building’s quirks, and the relationships that rooms have with each other.
Levels of Control
Controls are put in place to mitigate risk. As we work down the levels of controls, it becomes easier to achieve risk mitigation. However, cost and some other risks may increase. For example, it could be difficult to fix poorly sealed windows on a building due to the cost, however, the windows may last 50 or more years before replacing. Placing objects in sealed bags is a control at the object level, however the bag may have to be changed every 5-10 years which is labour and material cost. With bagging objects, we may inevitably trap pollutants inside or use plastic that off gases, thus increasing risk. For larger collections, it would also be a large and prohibitive expense to bag every item. Therefore, when building an IPM plan and preventive strategy, attempting to minimise risks on a higher control level will assist more objects for less money.
Location
Location is what is happening outside of your site. It can be difficult to put in controls at this level that directly pest control outcomes. However, understanding where large scale risks may be generated from can inform an IPM plan to implement certain types of risk mitigation and therefore be more effective.
What sort of location is your museum or collection in? This will dictate what sort of pests you will find in your collections
Urban: more pests associated with human habitation e.g., mice, cockroaches, silverfish
Natural/parks: more pests associated with plants e.g., carpet beetle, borer
Rural/Agricultural: more pets associated with mass plantings and farms, e.g., crickets
Site
The location of the site, and other amenities on site will influence pest risk and the ability to put in controls for risks.
The site is defined by the border, either legal, fence, vegetation etc. Some sites may have more difficulty in defining the boundaries, however, it is best to stop where an organisation has administrative control. Influences outside of administrative control can be thought of as location.
Building
Each building or cluster of buildings may have its own levels of control. The building level of control is the last defence before a pest species can enter a building. It is crucial that controls at this level are maintained.
Room
The room can act as a barrier between pests moving around the building. It can be the space where people may want to concentrate their efforts in preventive conservation, however, looking at the above levels will result in stronger responses.
Object
Controls at this level can be difficult to maintain. However, microclimates, storage vessels etc, do provide an extra level of protection. If an organisation has a lot of people working in collections, such as volunteers, this may allow for this level of control to be utilised with minimal cost.
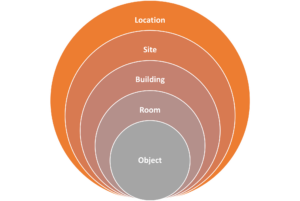
Figure 2: Visual key to understand the way the levels of control interact with each other.
Principles of IPM
The principles of IPM are Avoid, Block, Detect, Respond, and Review. These principles are used to generate an IPM plan, using the map and levels of control as a reference point. These principles were adapted from the 2019 book Preventive Conservation: Collection Storage, Integrated Pest Management for Museum Collections. Each principle will become a heading in your plan, which you can tailor to your institution, creating procedures in relation to each principle.
- Avoid: situations that support the ingress and development of pests. IPM Plan and preventing thinking.
- Block: pest ingress and systems that support their life cycle
- Detect: Regular monitoring of buildings, rooms, and objects; blunder traps, pheromones, rodent stations, inspections.
- Respond: Non-chemical methods of pest eradication. Targeted application of insecticides and rodenticides when appropriate.
- Recover: Review practices, understand sources, change, and build.
The steps should be attempted to be followed linearly, as they prioritise a non-chemical approach to controlling pests. Details of each step will be covered below.
Principle: Avoid
The principle of ‘Avoid’ is to eliminate the risk by avoiding situations that could help introduce pests into the building. Quarantining material before entering higher risk spaces is the most crucial step to eliminating pest risk to collections.
Quarantine
The separation of high-risk material before it enters the collection or display areas is crucial in reducing the risk of pest damage to the collection. Any person bringing any materials – organic or inorganic – into a collection area should be aware of the risk of transferring pests into the collection. There are three key steps in the quarantine process: separation, inspection, and treatment.
It is the responsibility of anyone bringing or storing material, including non-collection items, onto site that they follow these steps.
The following is a list of types of material that must undergo a quarantine process before being brought onto site due to their high risk of carrying museum pests specifically. This list is not an exhaustive list and best judgement based on the individual collection should be utilised:
- Cardboard
- Flowers and plants
- Collection material; organic and inorganic
- Packing material for collections
- Exhibition material
- Material that will be brought into or stored in collection areas
- Material that has been deemed high risk by Collection Managers or Collection Care.
Separation
Separation procedures occur when materials (collection and non-collection) are at risk of harbouring pests. The procedures are important for bringing material into or between high-risk zones such as collection stores and exhibition spaces.
Building plans assist with identifying points of delivery for items that have been identified as requiring quarantining. At each point identify the appropriate steps to take for identified items. Dispose of high-risk material in quarantine spaces and only bring what is necessary into the building
Disposal bins should be placed close to quarantine areas to allow for disposal of high-risk material, to prevent it being brought into the museum. Signage that alerts and reminds people of the nature and use of the area, especially the high risk of infestation, can be placed on walls near these disposal sites.
Inspection
Trained staff should conduct inspection of high-risk material. Inspection should be used to identify active/inactive pest activity and assess the risk, and devise mitigation strategies. If a risk mitigation strategy is implemented, then a record should be made, and appropriate staff notified.
- Visual inspection of material
- Use of extra lighting and magnifiers as necessary
- Treatment necessary or sufficient risk mitigation strategies in place
- Notification of appropriate staff
Treatment
The treatment step will depend on a museum’s capability balanced with the risk of pest infestation. The IPM plan should identify the risk the introduction of this material would be to certain spaces and whether the material is required to undergo treatment. If the decision is made to undertake a quarantine treatment, this should be done in a designated quarantine space, again ensuring this occurs before the material has a chance to enter a collection area.
The treatment options are outlined in the ‘Respond’ section below. Non-collection material such as flowers, may undergo chemical treatment such as pyrethrum spray.
Lighting
Lighting outside a building will often attract insects that are active at night. While most museum pests are not attracted to light, any insects that enter the building may become a source of food for other pests (these fall into the ‘predator’ and ‘nuisance’ category of pests). Lights near the building should be directed away from external walls, so as not to act as a light-trap for insects, and fitted with globes that are less attractive to insects (e.g., lights with low sodium output, such as yellow sodium globes).
Due to light damage, it is best practice to only have lighting on in collection areas as necessary, and this will also reduce some pest activity – e.g., large moths. Lighting decisions inside buildings should be balanced with understand pest biology, as many museum pests will favour a dark environment – e.g., carpet beetle. The known damage of light and cost of running power will impact any decision to use lighting spaces as a pest prevention measure.
Another pest considerations with lighting is that they form a source of heat for insects and some pests may be attracted to the lighting source. Having light sources outside of display cases and away from a plinth will reduce heat and may make the area less attractive to insects.
Principle: Block
The block principle forms the ‘engineering and administrative controls’ in risk management. The principle is minimising pest ingress into spaces and around buildings. This can include making environments less appealing for pests when they are looking for a place to live and breed.
Physical Control
Assess your building for entry points by insects even very small or medium sized pests. Check windows, doors, roof spaces, eaves, secret tunnels, vents, between floorboards.
It is difficult to ensure all entryways are sealed against pests. Many beetle pest species in Museums can fit through holes above 0.7mm and common mice specimens can enter through holes that are mere centimetres wide. Therefore, being thorough in inspections and attempting to secure all perimeters is ideal. Further details can be found in Pest Prevention By Design, pg. 15 (Version 11/28/12).
Windows
If not fully sealed when closed, you can use draught seals or rubber seals that create a flush/airtight seal. If windows need to remain open for ventilation, use screens – ensuring they cover all entry ways. Use a flyscreen that is appropriate for the location of the building. Thrips, a common garden pest but not to collection, are only restricted by a mesh size of 0.182mm or 80 mesh gauge (Pest Prevention By Design). This size is more difficult to maintain, therefore, a 2.03mm or ten mesh gauge might be more achievable. Know the mesh size and what insects will be able to pass through the material you have, and whether this is a tolerable risk to the collection. Fine mesh may have an impact on airflow and if airflow is important for mould prevention, then this needs to be considered as well.
Screens can also be applied to vents, and grills into buildings. Similar rules to above would apply. It is important to note that if the opening is used for airflow for a HVAC, then a minimal airflow would apply. Fine netting is a great way to apply a cover over a vent or other opening that needs to stay open for ventilation purposes but could be a point of ingress.
Testing of nets impregnated with an insecticidal additive has been noted which may give even greater protection from certain insect attack, however testing is preliminary at this stage.
Doors
Doors follow similar principles to window coverings. If the door is required to stay open then consider and use the information about screens above, an alternative would be the installation of an air curtain, which can provide a high effective rate in preventing pest ingress. Ensure that all seals are flush to the surface to provide effective risk control.
External doors require higher levels of seals, than internal doors. Seals also prevent the ingress of dirt and debris into the collection area, which not only reduces pest risks but reduces dust on objects. External doors will require a rubber seal that runs flush to the ground, this provides an extra layer of protection and minimises water ingress.
All doors to collection areas should be fitted with brush seals and/or draught stoppers to prevent entry of insects and will also help reduce the accumulation of dead insects and other debris around doorways. This debris can attract other insects; therefore, regular cleaning will reduce the attraction of pest through the building.
Weatherstripping with foam or rubber seals can add extra protection if pests are a significant risk to a collection area. Understanding the pests that are being controlled is important in deciding if this is necessary. Silverfish are unable to crawl on slippery surfaces, and therefore a strip along the edge of a door will do little to prevent access. However, it may prevent against moths accessing.
As an interim solution to seals if installation is difficult. Use other objects to block access, such as sandbags. This may slightly reduce pests accidentally accessing the space – however, if a pest is determined then they will be able to still get through.
Materials
Collection furniture and exhibition mounts could be made of material that inhibits pest access, e.g., slippery surfaces such as powder coated metal and acrylic may inhibit crawling pests being able to climb onto and access collections. Of course, this will not inhibit flying insects from access.
Environmental control
The AICCM Environmental guidelines can be found here. Below is discussion about the considerations from a pest risk perspective on the influence of the environment on pest activity.
The environment will influence insect reproduction cycle and as such its control is important to monitor (along with other preventive reasons). Technically maintaining a perpetually low temperature below 15 degrees Celsius will discourage most insect activity. However, in most situations this is not possible to maintain in collection areas especially where people work, and it will require additional constant humidity control. A low humidity <40% is also a way to discourage insects, however this is not an appropriate temperature for some materials, especially organics. As such, using temperature and humidity to discourage pests has limited application.
It is better to keep in mind that insect infestations are associated with:
- Temperature fluctuations
- Sustained high humidity
- Nearby water sources (puddles, drains and gutters)
How to minimise these effects on your environment will depend on how much control you can have over your space. If it is difficult to control an entire area where collections are held, consider microclimates or anoxic low humidity encapsulation for objects that require extra protection. Breathable non-organic materials like spun polyester work well if you can create a full seal.
Workplace Cultural Control
Once access is sealed you can start looking at whether your furnishings or materials indoors might encourage insects. Collections should be held separate to the everyday business of the museum – e.g., offices, labs, workshops, public areas, lunchrooms, cafes. These are high volume visitation areas that involve the use of many pest attractants e.g., food, wood, packaging. These high-volume spaces are difficult to control and seal. Furthermore:
- Collections should be housed internally e.g., accessible by doors that do not lead directly outside – one more barrier to get through is always helpful, and this acts as an environmental fluctuations buffer also
- Furniture made of wool, feather, untreated wood – should be avoided or treated before being brought into a space
- Bins should have lids, emptied regularly, food waste bins should be kept far away from collection areas
Deliveries: when deliveries arrive, they may be wrapped in cardboard and other organic packing material – these are high pest risk materials and need to be remove from site as quickly as possible.
Policies: policies for how food is stored and delivered to the site, policies about how deliveries of flowers and raw plant materials are brought into the building. Procedures help people learn and know who to contact and what to do when something enters the museum – whether that is an object, specimen, something for the shop, or a stationery delivery.
Housekeeping: A simple strategy to control pest infestations is keeping a neat and tidy store and undertaking regular deep cleans. When you undertake consistent cleans you:
- Remove sources of food such as dust, leaf matter, or other material that might have accumulated e.g., brought in by human traffic over time
- Use the time to undertake spot inspections
- Find and get rid of any packing material or other materials no longer in use that may have snuck into the store
Cleaning includes:
- Wiping surfaces of dust
- Cleaning underneath shelving and furniture with void spaces
- Cleaning windowsills and near doorways
- Removing accumulated debris or things forgotten or left behind in the store
Chemical deterrents
Chemicals can be used to mask or deter pest ingress. Some of these chemicals have an impact on human health or to objects. They should be used with care and consideration. Some natural alternatives that are local to the organisation may be appropriate for collection use. Care should be taken when placing dried flowers, spices, or timber in collections that they do not become a pest source themselves. Some common deterrents include:
- Camphor
- Naphthalene
- Essential oils
- Cedar wood
Principle: Detect
Detection allows for pest prevention through early detection of pests in areas, environmental conditions (particularly microclimates) and ingress points. When conducting inspection routines, it is important to have the following:
- Torch
- Sticky traps
- Logbook and pencil
- Insect identification kit or pictures
- Small plastic vials and tweezers
- Building map with traps marked
- Plastic bags
- Hand lens
Insect Biology
Learn what insects and invertebrate pests are likely to live in your area. Learn their seasonal habits and when they will be breeding/egg laying. Tips include:
- Befriend a local entomologist, mammologist, ecologist
- Join MuseumPests Pestlist (https://museumpests.net/join-the-pestlist/)
- Use free online resources to identify insects e.g., iNaturalist, MuseumPests
- Knowledge of non-pest specimens is sometimes helpful as a diagnostic tool, so having a wide knowledge is useful
Learn what food they like to eat – Protein? Cellulose?
Understanding feeding guilds, insects can be divided by the food that they eat. Common insect feeding guilds are:
- Necrophages: Insects that feed on carrion (dead animals). Understanding where these insects will feed in cycle is important. Museum objects being older and having less moisture content, the necrophages that are museum pests typically arrive at the end of the breakdown process.
- Granivore: Insects that eat seeds including grain. These pests are usually more associated with agriculture. However – seeds can often part of museum collection objects
- Xylophages: Insects that eat and decompose timber. Many pests will have a preferred moisture content in the timber they like to eat, and this is valuable information to understand when detecting pests
Other non-Museum pests:
- Leaf miners: Insects that eat leaves on growing plants
- Frugivores: Insects that eat fruit
- Granivore: Insects that eat seeds including grain. These seeds often make up collection objects
- Sap Feeder: Insects that eat the fluid within plants. Only will feed on green timber or alive plants
- Root Feeders: Insects that consume the roots of living plants
- Stem Feeders: Insects which consume the stems of plants including trees. Many non-museum pest borers are a part of this guild.
Based on an insect’s feeding guild and biology, the risk of the pest can be determined.
- Pest: Can feed on Museum collections
- Nuisance: A chance visitor, their presence may provide food for other insects or droppings can cause damage.
- Predator: Eats other insects or is parasitic.
- Indicator: Indicates an environmental problem. This category is often blurred with some of the ones above and can be used in conjunction with the others.
This list is not extensive, and it is important to understand the pests which are in collections and what they typically feed on this will allow for investigation of their source.
While not covered in this text, extra reading should be done to understand insect mouth parts, legs, and locomotion. Having an important grasp on these elements of biology, in particularly of the local insect population will allow for more comprehensive analysis of data.
Don’t forget about Vertebrate pests: Rodents big and small, lizards, cats, seagulls, monkeys, squirrels, other vertebrates.
Rodents can and will eat materials covered by all food guilds and should be considered significant pests. They also collect materials such as paper and textiles to line their nests which they usually create in walls within easy reach of food sources. These nests are often imbued with rodent urine.
Most rodents also do not have specific bladder control and will trail urine wherever they pass. This is a concern for humans as some rodents (including squirrels), can carry serious illnesses such as Hantavirus. For collections there is another risk: as per most vertebrates’ rodent urine is very acidic and will cause acidic burns to all susceptible materials – from paper to stone.
Observation
Turn everyone into a pest inspector/pest spy. Teach people what pests look like and what sort of insect activity you need to be told about. Front of house staff are perfect for this role. They will also be the main people who will ensure doors and windows stay closed throughout the day during visitor hours etc., so it is essential to pass on basic IPM knowledge to what will be your first line of defence against emergent pest outbreaks.
Sticky Traps
Sticky traps are a monitoring device and not a pest eradication method. Sticky traps are considered blunder traps – by themselves they do not attract pests but rely on being in the right place at the right time to indicate what insects or small invertebrates are walking around your collection area.
Sticky traps should be placed around a room to monitor particularly susceptible areas such as doorways, windows, and other potential ingress points around the collection store. They can also be placed around or near objects that are highly susceptible to insect attack or are highly significant.
Pest traps should be checked regularly – I.e., as much as possible considering that this is a process that is involved and can take a bit of time. A good guide is to check at least once within a breeding cycle of a potential pest.
When checking traps, the insects are counted along with information of the trap’s location and date it was accessed. The reason for collecting data from traps is to recognise when a pest infestation is by recognising spikes in insect numbers. It can be an indication whether entry points are properly sealed against insects, and whether one object or area is undergoing an infestation situation. Ideally, this can help you track down the exact objects being attacked during a pest outbreak.
Some sticky traps can be baited either in pellet bait that will attract certain insects.
Sticky traps will occasionally trap vertebrates, this is an unwanted effect and can be cruel to the animal. Plastic covers that limit access points to traps can minimise this. However, covers may also reduce the number of insects caught and this can be considered in the data analytics.
Pheromone Traps
These traps serve a similar purpose but by employing a different tactic. Pheromone traps will attract male insects of a particular species due to the inclusion of pheromones in combination with a sticky trap surface. These pheromones can be applied to a sticky surface in the form of tablets attached to the sticky part of the trap, while some are impregnated into the sticky surface already.
As mentioned, these traps are usually only baited with female pheromones I.e., they will only capture adult male specimens. This is important – these traps are more successful in terms of trapping and killing insects as they do not rely on accidental blundering of the insect, but they are not trapping the specimens that are the laying the eggs (female), and they are not the insects that are physically eating the collection because these traps do not attract larval stages.
As such just like sticky traps, these traps can alert you to an explosion in the insect population, and potential ingress points. They can also help you detect the direction or flashpoint of your infestation down to one area of the collection or the one object being a source of infestation.
Catch traps
There are several insect-catching traps on the market; they are generally simple design that attract and encourage an insect to get into a pit or enclose that it cannot escape. Their effectiveness has come under question and will be based on how attractive the bait/pheromone is.
Termite and wood borer monitors
Timber monitors typically have a timber source inside that can be inspected at regular intervals to check for terminate activity. They can be placed in the ground or in walls or can simply be a box of timber in a container. Understanding the local termite and wood borer populations favoured timber species and moisture content will allow for better bait choice. A local pest practitioner who regular deals with termites will be able to assist you.
Termite detection dogs have also been found to be effective in the early detection of termite activity. A trusted private practitioner may have a trained animal that can be hired by the organisation.
Another visual inspection guide is placing paper underneath objects to catch any debris, this will allow for seeing if fresh frass is being created from exit holes. This is a low-cost option that can be included in routine inspections. Additionally, understanding that termite infested timber sounds hollower when knocked will allow for self-inspections of buildings and objects. Unless the person inspecting does this often, it may not provide accurate results.
Rodents
Rodents can be trapped or baited. When a rodent is baited this usually means ingesting a poison. Most poisons kill slowly so it is essential to understand that this may lead to bodies being trapped dead in small spaces after being baited.
Common Rodent traps on the market:
- Baffle traps
- Bat boxes
- Traditional baited mouse traps
- Sticky traps
- Catch and Release traps
It is important to think about what sort of rodent trap an organisation wants to use in a space and overall. Different traps can be used in different areas depending on needs.
Baited baffle traps and simple box bait traps work by introducing a poison into the rodent. The bait is highly effective at being tempting to rodents and a high kill rate. The poison does not instantly kill the rodent but affects its body to encourage internal bleeding eventually leading to death. This causes two things: one, a prolonged death – this may not be appropriate depending on your personal belief systems. Two: a death in an unknown secondary location. You cannot know where the rodent will die – this can be inconvenient as they may die in the roof space, inside a wall, etc. It is important to regularly check poison baits, as they can become a source of museum pests if left undisturbed for long periods of time.
Baiting mice and rats also leads to poisoning of local animals such as owls and eagles that predate on rodents and can become sick and die if they are eating effected rodents.
Catch and release traps can allow you to catch a rodent and release it far from your collection, however if the main attraction of this method is the human aspect, please consider the amount of time the rodent will be trapped and how much pain, stress, and death this could lead to. Catch and release traps should be checked every four hours otherwise the trap can lead to death of the animal.
Traditional mice and rat traps cause instant death and in terms of extermination this is the quickest and most humane method. However, these traps must be checked regularly otherwise the carcass will itself become a food source for proteinaceous pests such as carpet beetle.
Trap placement
Several blunder traps should be placed in each monitored area, in locations where insects are likely to meet them (i.e., near doors, wall edges, window ledges and corners of rooms). These traps should be numbered, and their location recorded on a map.
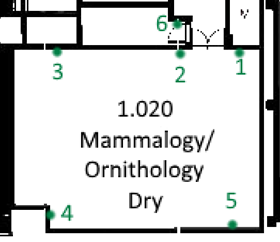
Figure 3: General; routine inspection traps around ingress and strategic locations
Additional trapping can be carried out when a high risks of pest activity is noted, or active pest activity occurs. There are three suggested styles of additional trapping that can be implemented to determine the level of pest activity, location, and ingress patterns. The additional traps can contain a pheromone lure if a specific species is being targeted. Care should be taken when using pheromone lures as to not attract pest activity into collection areas which will sway the data analytics.
- Extensive: Grid pattern across store: Placing blunder traps at regular interval forming a grid across a space
- Targeted: Additional traps placed near targeted collection items
- General: Additional traps placed on all possible points of ingress into a store
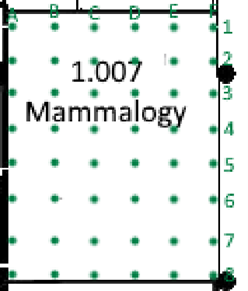
Figure 4: Extensive trapping; gird formation with numbering
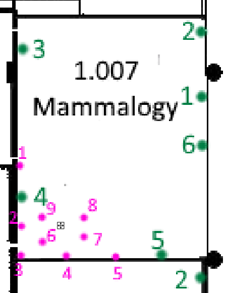
Figure 5: Targeted; specific area around risks
Recording data
IPM records should be recorded in a uniformed approach, easily accessible and centralised.
Required records include:
- Trapping data
- Additional incident reports
- Timetables of treatments and when they were carried out
- Reports from pest contractors
- SDS’s and other information on treatment methods
Trapping data
Data collected from traps should be recorded in a standard approach that is regularly updated depending on the organisation’s requirements. This may be at quarterly or monthly intervals. Software companies such as Conserv (https://conserv.io/software/ or https://zpesttracker.com/ allow for information to be added directly into a database. Simplified options can be a printed table, or information can be added to a spreadsheet and user analysis occurs afterwards.
At a minimum the data recorded should be:
- General Trap Location, e.g., Building
- Room
- Trap Number (recorded on map and kept consistent)
- Date of inspection
- Common name of insect, scientific name is optional
- Life stage (egg, larvae, adult, or moult)
- Number of that type of pests
- Risk factor; Pest, nuisance, predator, indicator
Further information can be found on MuseumPests, accessed here.
The other systems which attempt to equalise some of the variation in data collection allowing for better cross store comparison was created by Baars and Henderson. The articles with details and a spreadsheet template can be found here and here.
Principle: Respond
Responding to infestation is crucial. Pests pose different risks to collections, and this will affect the timing and level of response. Understanding the insect biology and if the insect or pest will eat the collection is important in making this decision
Finding an Infestation
Finding an object with signs of infestation is common – most museum collections, private collections, galleries etc. will have some signs of previous pests on objects. Old holes in future, dry husks of juvenile carpet beetles, frass and holes in clothes are a common sight in collections.
It is difficult to know whether the damage you find is new or old (unless you find a living insect actively eating, or the exuviae appears particularly fresh and malleable).
If you have:
- A photograph of the objects showing the damage, after which the material has been treated
- You know it has just returned from treatment
You can be confident that your evidence of pests is ‘old’ I.e., already treated and no longer a threat.
Otherwise, when you find an object that has a pest issue, undertake the following:
- Keep it where it is – do not move it unless necessary, this stops eggs etc dropping out of your object into surrounding areas
- Plastic wrap to encapsulate the object
- Deliver the object directly to the treatment area
Methods of Treatment
The choice in the method of treatment will be affected by firstly resources within your institution and secondly the material you are treating. The most common methods are:
Freezing
Low temperature treatments have been shown to be effective against a variety of insect species and across all stages of an insect’s life. The item to be treated is placed in a plastic bag (polyethylene), which is then sealed to prevent condensation build-up on the object upon thawing, alternately the item can be placed in a plastic tub that can be sealed. A buffering material, such as acid free tissue, can be placed in the container as well to ensure the moisture content of the environment remains stable and minimises the risk of water condensing on the interior surface. The item is then placed in a freezer for a pre-determined length of time (one to two week, depending on the object density). When considering treatment time, it is important to understand the density of the item and amount of material being frozen, the denser the item and tightly packed the longer the treatment time to accommodate for the longer period for the internal to reach required temperature. The freezer temperature should be operating at -30°C or lower to ensure complete mortality of all stages of insect life cycles. If a freezer can only operate at -20°C, then a two-to-three-week period should be used.
Method:
- Material protected from condensation using a polyethylene bag or sealed container
- If vulnerable material or non-absorbent material being frozen buffing agent to container
- Kept inside freezer for two weeks at -30 (length of time based on density of materials and air circulations around material, preferable for treatment time to be longer if unsure)
- If targeting a specific pest, it is advisable to research kills times for all life stages of that species.
- Removed from freezer and left to reach room temperature inside bag or container, condensation should be gone by the material is removed from container.
- Treatment recorded.
Anoxia
Anoxia, low oxygen is useful for objects which are moisture or temperature sensitive. It is completed by either absorbing all the oxygen in an enclosure or replacing the oxygen with an inert gas.
Items can be placed in a high gas barrier bag along with oxygen scavengers and heat sealed, this will create a low oxygen environment which will kill insect activity. Death is caused by an insect spiracular being forced open due to low concentration of oxygen and then subsequently the dehydration of the insect. This method can be unreliable due to faults in scavengers or the bag, however, when done correctly it can be effective. It should be noted that scavengers often reduce oxygen through an exothermic reaction, and this may cause harm to some sensitive materials if placed directly next to the scavengers. There is also the harm that leaving material in bags for extended periods (years) may create, microclimates and a build-up of gases that may attract more pests. The following are guidelines for current practices, however, since this method uses commercial products testing of materials and controls should periodically be conducted.
Method:
- The item to be treated is placed in a bag made from a high gas barrier film (a film with a low rate of transmission for oxygen) such as a ceramic-evaporated transparent film or aluminium film bag.
- Required amount of oxygen scavengers are added to the bag, with a barrier between the item and the scavenger.
- Mitsubishi Type K – absorbs oxygen and ‘corrosive gases.
- Mitsubishi Type A – absorbs oxygen, ‘corrosive gases and moisture.
- Ageless Z2000 – absorbs just oxygen.
- The film bag is heat sealed shut, it is preferable to do a double seal to minimise risk of errors.
- Oxygen level is measured using an oxygen meter (0.5% is target). If an oxygen meter is unavailable, measuring the loss of volume of a bag will give a rough estimate or oxygen indicator ‘pills’ are available.
- The time it takes to eradicate pests depends on the species being targeted, it can range between 4-20 days. However, to ensure all stages of an insect have been killed it is recommended to keep the item at below 0.5% for a minimum of three weeks
- Bag is opened after this period.
- Treatment is recorded.
Heat Treatment
Heat treatment for scientific material is commonly used throughout the world with success in eliminating pests. There are limitations on which material can tolerate higher temperatures, therefore, unless specified, material should not be treated in this manner. The advantage of this method is that it is the quickest method with the same effectiveness as low temperature.
- The treatment is more limited in its applicability of materials; however, it is quick and effective.
- Requires specialist equipment.
- No plastic wrapping of materials required.
Method:
- Check items to ensure that they will be resistant to the heat required for the treatment.
- Place items in a chamber that controls the temperature and humidity – see equipment section for details.
- Place temperature probe in core sample, timber of an appropriate size comparable to the thickest object.
- Ensure that other temperature and humidity sensors are placed around the chamber.
- Heat chamber up to a core temperature of 55C and hold temperature at this minimum for a period of 4 hours while holding the RH (Relative Humidity) at 55%.
- Steadily bring the chamber temperature back to ambient while keeping the RH stable.
- Remove items from chamber and treatment is complete.
- Total time for treatment can be as little as 24-48 hours, this will depend on the density of the material being treated.
Finding an infested room
Sometimes, pests can infest an entire room. When signs of an infestation are found in an entire room, the whole collection within should be treated for pest infestation. Non-organic materials can be inspected and cleaned/brush vacuumed.
A method of treating entire room of infected material includes:
- Bagging up everything and treating individually, only returned once the room itself is treated, cleaned, and access to insects managed
- Raising or lowering the temperature and humidity in the room
Insecticidal spray
Pesticides should be considered the last defence in an IPM plan. Most insecticidal sprays will require some licensing to apply, and this will depend on laws in your local area. It is always best to understand what restrictions are in place regarding chemical usage and discuss them with your pest management professionals.
The use of insecticidal sprays will kill several insects in a known area at a particular time i.e., a pesticide is usually sprayed along the perimeter of a room. This insecticide will remain active for a defined amount of time and kill anything that walks into this path. The spray is done away from objects, however, to ensure none of the solutions is carried onto objects. An Insect Growth Regulator is a spray that is also applied to the perimeter of a room and inhibits insects growing, eventually leading to death.
‘Natural’ spray itself is likely to be pyrethrum-based or nicotine based. There are other ‘harsher’ chemicals can be from a variety of chemicals. Many have their own local restrictions, due to their environmental and health hazards. If your pest provider suggests a chemical, make sure to research it beforehand and understand its affects.
In general, the perimeter spray method only kills insects that are already in the store or that will enter the store very shortly after the treatment. It will not affect pests that are infesting objects internally. While there are some shortcomings, spraying is the quickest way to remove several insects at once from an area. After a spray it is a good idea to monitor your building to see what insects appear dead in the area. These should be removed and if possible recorded.
Pyrethrum
Usually an oil-based spray, that has a short active time. The spray is based on chrysanthemum flowers and is usually only sprayed directly on flowers or non-collection items that are not in contact with the collection.
Pyrethroid and Neonicotinoids
Pyrethroid and nicotine: industrial chemicals that are based on natural insecticides. Usually, water-based but can also be distributed in an air fog. Their advantage is that they are much more stage than complete natural options and can provide a last effect for a few weeks if left undisturbed.
Diatomaceous Earth
Silica-based powder that can be applied to cracks and crevices. It can be distributed as a powder or in a paste. However, it will only be effective when dry. It has been shown to be effective in controlling moisture in cracks in buildings, however, the pest qualities when wet are less effective.
Principle: Recover
The recover step is an administrative control to ensure that the above principles are effective for the collection. The aim of recover is to determine if there is a known access point where a pest may have originated and if the IPM plan is sufficient in reducing risk. Once this is determined, an improved strategy to prevent a future outbreak of pests from occurring can formulated.
Determine the cause of the pest infestation. Inspection of entry points, nearby objects, and workplace procedures is necessary. Signs of pest entry can be from:
- Dust and dirt debris
- Visible hole
- Water leak or staining
- Birds’ nests
- Spider webs
- Packaging material or housing materials for objects.
Deep Clean
If it is possible to do so, after a pest treatment – undertake a deep clean of the room, ideally before a treated object is returned. That means your newly treated object is returning to an environment that is less likely to harbour more pests that will attack the material again. If you do not treat the area, you will soon treat the same or another object quickly.
Review
Reviewing the IPM plan regularly and after an infestation is crucial in determining its effectiveness. If the source of an infestation or high pest numbers in traps can be determined, this should be included in the plan. As part of the review strategy, the organisation should map out key stakeholders and areas of responsibility beforehand. Having these stakeholders aware of pest risk beforehand allows for easier communication of pest prevention needs.
Depending on the organisation’s size, identify the following stakeholders and ensure that engagement occurs during the creation of an IPM and during the recovery stage.
- Collection staff
- Conservation
- Facilities or building management.
- Grounds staff
- Cleaning staff
- Exhibition staff
- Executive staff, depending on the level of infestation.
Before an infestation occurs, the role of these staff in an organisation’s response to a pest infestation should already be mapped out in the IPM plan.
Other Resources
ICC – Pest Prevention Building Guidelines
IPM Building and Site Checklist
References
Strang, T, Jacobs, J & Kigawa, R (2019),’Integrated Pest Management for Museum Collections’, in Elkin L and Norris C (eds) Preventive Conservation: Collection Storage, Society for the Preservation of Natural History Collections,New York, USA.
Geiger, C & Cox, C (2012), Pest Prevention by Design: Authoritative Guidelines for Designing Pests Out of Structures, San Franciscio, USA.